|
Bioprocess Equipments and Global Regulatory Validation
Fermentor /
Bioreactor / Cip system /
Sip system /
Process vessel / Filtration system
/ Crystallizer / Bioinactivation system /
Training / Automation solutions
/ Bioprocess solutions /
Regulatory services
https://en.wikipedia.org/wiki/Bioprocess_engineering
https://en.wikipedia.org/wiki/Biological_engineering
https://en.wikipedia.org/wiki/Bioprocess#Upstream_bioprocessing
https://en.wikipedia.org/wiki/Bioprocess#Downstream_bioprocessing
https://en.wikipedia.org/wiki/Bioreactor
https://en.wikipedia.org/wiki/Fermentation
https://en.wikipedia.org/wiki/Sterilization_(microbiology)
https://en.wikipedia.org/wiki/Clean-in-place
https://en.wikipedia.org/wiki/Pressure_vessel
https://en.wikipedia.org/wiki/Filtration
https://en.wikipedia.org/wiki/Cross-flow_filtration
https://en.wikipedia.org/wiki/Water_purification
https://en.wikipedia.org/wiki/Crystallization
https://www.solidswiki.com/index.php?title=Crystallizers
https://en.wikipedia.org/wiki/Biomedical_waste
https://en.wikipedia.org/wiki/Food_and_Drug_Administration
https://www.pda.org/

Bioreactor |
Bioprocess
engineering,
also biochemical
engineering,
is a specialization of chemical engineering or Biological engineering,
It deals with the design and development of equipment and processes for the
manufacturing of products such as agriculture, food, feed, pharmaceuticals, nutraceuticals, chemicals,
and polymers and paper from
biological materials & treatment of waste water. Bioprocess engineering is a
conglomerate of mathematics, biology and industrial design,and consists of
various spectrums like designing of bioreactors,
study of fermentors (mode of operations etc.). It also deals with studying
various biotechnological processes used in industries for large scale production
of biological product for optimization of yield in the end product and the
quality of end product. Bioprocess engineering may include the work of
mechanical, electrical, and industrial engineers to apply principles of their
disciplines to processes based on using living cells or sub component of such
cells.[1].
|
Biological
engineering
Biological engineering,
or bioengineering/bio-engineering,
is the application of principles of biology and the tools of engineering to
create usable, tangible, economically viable products.[1] Biological
engineering employs knowledge and expertise from a number of pure and applied
sciences,[2] such
as mass and heat transfer, kinetics, biocatalysts, biomechanics, bioinformatics,
separation and purification processes, bioreactor design, surface science, fluid
mechanics, thermodynamics,
and polymer science. It is used in the design of medical devices, diagnostic
equipment, biocompatible materials, renewable bioenergy, ecological engineering,
agricultural engineering, and other areas that improve the living standards of
societies.
Sub-disciplines
Depending on the institution and particular definitional boundaries employed,
some major branches of bioengineering may be categorized as (note these may
overlap):
[7]
Bioprocess engineering
Environmental
health engineering
Human-factors
engineering
Biotechnology
Biomimetics
Bioelectrical
engineering
Biomechanical engineering
Bionics
Bioprinting
Biorobotics
Systems biology
|

A ribosome is
a biological machine that
utilizes protein dynamics
|

A clean-in-place unit on display at the World of Coca-Cola in Atlanta
|
Clean-in-place (CIP)
is a method of cleaning the interior surfaces of pipes,
vessels, process equipment, filters and associated fittings, without disassembly.
Up to the 1950s, closed systems were disassembled and cleaned manually. The
advent of CIP was a boon to industries that needed frequent internal cleaning of
their processes. Industries that rely heavily on CIP are those requiring high
levels of hygiene, and include: dairy, beverage, brewing, processed foods, pharmaceutical, and cosmetics.
The benefit to industries that use CIP is that the
cleaning is faster, less labor-intensive and more repeatable, and poses less of
a chemical exposure risk. CIP started as a manual practice involving a balance
tank, centrifugal pump, and connection to the system
being cleaned. Since the 1950s, CIP has evolved to include fully automated
systems with programmable logic controllers,
multiple balance tanks, sensors, valves, heat exchangers, data acquisition and specially designed spray
nozzle systems. Simple, manually operated CIP systems can still be found in use
today.
|
|
Filtration is
any of various mechanical, physical or biological operations that separates
solids from fluids (liquids or gases)
by adding a medium through which only the fluid can pass. The fluid that passes
through is called the filtrate.[1] In
physical filters oversize
solids in the fluid are retained and in biological filters particulates are
trapped and ingested and metabolites are retained and removed. However, the
separation is not complete; solids will be contaminated with some fluid and
filtrate will contain fine particles (depending on the pore size, filter
thickness and biological activity). Filtration occurs both in nature and
in engineered systems;
there are biological, geological,
and industrial forms.
For example, in animals (including humans), renal filtration removes waste from
the blood,
and in water treatment and sewage treatment,
undesirable constituents are removed by absorption into a biological film grown
on or in the filter medium, as in slow sand filtration.
|

Diagram of simple filtration: oversize particles in the feed cannot
pass through the lattice structure of the filter, while fluid and small
particles pass through, becoming filtrate.
|

The international symbol for biological hazard.
|
Biomedical waste is any kind of waste containing infectious (or potentially infectious) materials.[1] It may also
include waste associated with the generation of biomedical waste that visually
appears to be of medical or laboratory origin (e.g., packaging, unused bandages,
infusion kits, etc.), as well research laboratory waste containing biomolecules
or organisms that are mainly restricted from environmental release. As detailed
below, discarded sharps are considered biomedical waste whether they
are contaminated or not, due to the possibility of being contaminated with blood
and their propensity to cause injury when not properly contained and disposed
of. Biomedical waste is a type of biowaste.
Biomedical waste may be solid or liquid. Examples of infectious waste include
discarded blood, sharps, unwanted microbiological cultures and stocks,
identifiable body parts (including those as a result of amputation), other human or animal tissue, used bandages and dressings, discarded gloves, other medical
supplies that may have been in contact with blood and body fluids, and laboratory waste that exhibits the
characteristics described above. Waste sharps include potentially contaminated
used (and unused discarded) needles, scalpels, lancets and other devices capable of
penetrating skin.
|
Leading with Quality, Performance and Cost
 Our partners, the BIOZEEN Biotech is major
leaders in customized Bioprocess Equipments and Training for the
Biopharmaceutical communities, and has based on extensive expertise and process
knowhow for supporting companies and governments for manufacturing medicines and
biotherapeutics ranging from MABs to vaccines, insulin, plasma, enzymes and APIs
at every stage of production life cycle globally.
Our partners, the BIOZEEN Biotech is major
leaders in customized Bioprocess Equipments and Training for the
Biopharmaceutical communities, and has based on extensive expertise and process
knowhow for supporting companies and governments for manufacturing medicines and
biotherapeutics ranging from MABs to vaccines, insulin, plasma, enzymes and APIs
at every stage of production life cycle globally.
With the knowledge base of our promoters and diverse experience and expertise
pool, BIOZEEN has
• Engineered designs which are 65% energy efficient
• Delivered solutions which are 30% cost efficient
• Designed and engineered equipment demonstrating up to 6 times improved yield
• Empowered 1200+ professionals to serve the industry better and still counting
It is our privilege to have associated with premier biopharmaceutical companies
and government organizations belonging to this industry and enable them
manufacture lifesaving drugs at every stage of production life cycle, and has
globally built key customer partrnerships around the Europe, Asia, Americas and
Oceania providing result driven production infrastructure for their
bio-production needs.
Below is a snapshot of some of our customers :
http://www.biozeen.com/clientele
• Alfa Laval • Avesthagen • Bharat Biotech International Ltd • Biological-E Ltd.
• Biotechnology Consortium India Ltd
• Biovel Life Sciences Ptd Ltd. • Biovet • Brilliant Industries Ltd. • Cadila
Pharmaceuticals • Cambrex
• Center for Cellular & Molecular Biology • Claris Life Science Ltd. • Finlay
Institute
• Department of Animal Production & Health, Sri Lanka • CIPLA • Gennova
Pharmaceuticals
• Globion India Pvt Ltd. • Glenmark Pharmaceuticals Ltd. • Green Signal Bio
Pharma Pvt. Ltd.
• Goodwin Biotechnology • Government Pharmaceutical Organization, Thailand •
Indian Immunologicals Ltd.
• Intervet India Pvt. Ltd. • Institute of Animal Health & Veterinary Biologicals
• Invitrogen • IPL Biotech
• iScape Sciences Pvt. Ltd. • Jawaharlal Nehru Technical University • Kemwel
Biopharma • Lupin Ltd.
• LG Life Science • Packo Inox • Panacea Biotech Ltd • Reliance Life Science (P)
Ltd. • Serum Institute of India
• Shantha Biotechnics Ltd. • Smart Lab • Syngene • The Biovac Institute •
Universiti Putra Malaysia
• USV Ltd. • Vetal • Xcelris Laboratories Ltd. • Zenotech Lab Ltd.

BiOZEEN Fermentors are
opimized and customised as per your process needs. Innovaavely designed BiOZEEN
Fermentors are suitable for culivation of microbial culture – ultilizing robust,
industry – standard components for easy integration into any production
facility.
|
BIOZEEN Fermentor design & engineering parameters ensure :
• Minimum space requirement
• Optimized Fermentor design
• Range: 5L - 25000L (Customized to user requirements)
• Skid-mounted structure • Agitator (Top/Bottom mounted)
• Flexibility in configuration of Fermentor to meet the budgets
• Design compliance to ASME BPE and GAMP 5 guidelines
• Material of construction
• Parts in contact with the media: SS 316L
• Internal surface Ra<= 0.4, Electro-polished
• Single/double mechanical seal
• Easy access during operation and routine maintenance
• PLC control system with HMI or SCADA
• Configurable control loops
• Safety features to protect the batch in case of any component failure |
|
For optimal performance,
our
Bioreactor designs come with the following advantages:
• Minimum space requirement
• Optimized Bioreactor design
• Range: 5 - 10000 liters (Customizable to user requirements)
• Skid-mounted structure
• Mechanical/Magnetic - low shear Agitator (Top/Bottom Mounted)
• Gas mixing station
• Flexibility in Bioreactor configuration to meet the budgets
• Design conforms to ASME BPE and GAMP 5 guidelines
• Material of construction
• Parts in contact with the media: SS 316L
• Internal surface Ra<= 0.4, Electro-polished
• Single/double mechanical seal
• Designed for easy access during operation and routine maintenance
• PLC control system with HMI or SCADA
• Configurable control loops
• Safety features to protect the batch in case of any component failure .
|

Optimized and customised as per your process needs. Innovatively designed
BiOZEEN Bioreactors are suitable for cultivation of mammalian cell
culture–ultilizing robust, industry - standard components for easy integration
into any producction facility.
|

CIP (Clean-in-Place )Systems are designed for automatic cleaning ensuring
minimal use of water & time. BiOZEEN also manufactures docking station for CIP
of mobile systems that do not have integrated CIP function.
|
CIP system
has many benefits to the end user, some of them to mention are:
• Production down time between product runs can be minimized
• Cleaning costs can be reduced substantially by recycling cleaning solutions
• Water consumption is reduced as cleaning cycles are designed to use optimum
quantity of water Automated CIP system can provide full data logging for quality
assurance requirements
• Over and above BiOZEEN designed CIP system offers our customers the add-on
advantage, such as:
• Space conserving design
• Skid mounted (Single/Double tank system)
• Integrated mobile or split system (Volume dependent)
• Flexible range
• Varying configurations
• Minimal maintenance
• Vessel construction in SS 316L
• Requisite instrumentation to accord safety
• PLC control system with HMI or SCADA.
|
|
|
|
|
SIP
(Sterilization-in-place) Systems
sterilize mobile systems that normally do not
have the in-situ CLEANING WITHOUT VISUAL DISTORTION sterilization facility SIP
system (Sterilization - in - Place] caters to mobile vessels that normally do
not have the complete in-situ sterilization facility, unlike fixed equipment
that come integrated with sterilization capability.
All control valves required for sterilization are in built into the system. By
connecting the mobile vessels to the system, complete sterilization including
vacuum break can be achieved. Further the system comes with all the data related
to sterilization, which is essential for records and quality assurance.
BIOZEEN'S SIP system offers the following advantages:
• Skid mounted
• Corrosion resistant body and trim
• Self-draining design
• Material of construction SS 316L
• Sanitary process connections BIOZEEN also manufactures integrated docking
station for CIP and SIP functions.
|
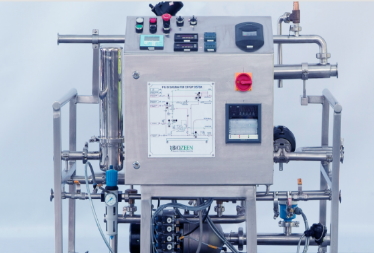
SIP (Sterilization-in-place) Systems sterilize mobile systems that normally do
not have the in-situ CLEANING WITHOUT VISUAL DISTORTION sterilization facility.
|

BiOZEEN Upstream and Downstream Processing Systems are designed and built to
meet the process requirements at all stages of the manufacturing process life. •
Media Preparaon
• Buffer Preparaon
• Mixing/ Blending
• Collecon/Holding
• Harvesng
• Precipitaon
• Detoxificaon
• Customized Systems.
|
Process
Vessel is designed and built to meet the process requirements at all stages
of the process life cycle including and not limited to:
• Media preparation
• Buffer preparation
• Mixing / Blending
• Harvesting
• Collection/ Holding
• Precipitation
• Detoxification
• Pressure vessels
The Process Vessel design & engineering ensures:
• Minimal space requirement
• Easy maintenance
• Easy integration with plant facility
• Flexible range
• Flexible configuration
• Compliance to ASME BPE guidelines, ensuring cleanability, drainability and
sterilizability
The design specifications for the Process Vessel includes:
• Material of construction
• Parts in contact with the media: SS 316L
• Internal surface Ra<=0.8, Electro-polished
• Components selected are of reputed makes
• Agitation System (Mechanical mixer/ Magnetic) - Top/Bottom Mounted
• Design conformity to ASME BPE and GAMP 5 guidelines
• PLC control system with HMI or SCADA .
|
|
Filtration System play a vital role in Biologics & Biopharmaceutical
industry.
Their application range varies from pilot scale to full-scale production. Some
of the applications in a Biopharmaceutical industry include:
• Vaccine and conjugate concentration
• Recovery of antibodies or recombinant proteins
• Fractionation of protein mixtures
• Clarification of fermentation broths
• Cell broth clarification, concentration
•• Blood plasma fractionation and purification
Our design features for Microfiltration Ultrafiltration and Hollow Fiber
Filtration System provide you the following advantages:
• Skid mounted, Space conserving, Easy maintenance
• Fixed/Mobile, with or without vessel
• Automated flux measurement to check cleanability
• In-situ sterilizable with or without cassette
• Optimized stabilization time
• Provision for online membrane integrity test
• Minimal hold up volume and Maximum product recovery
• Cassette holders offered to suit different membrane manufacturers
• Automated trans membrane pressure maintenance
• PLC control system with HMI or SCADA .
|

RRange of filtration system include microfiltration, ultrafiltration and hollow
fibre filtration system which can efficiently handle separation of solutions
containing biomolecules or particles such as viruses, bacteria or cellular
material.
|

A Crystallizer comes to use in the production of an Active Pharmaceutical
Ingredient (API). For the manufacture of a desired API, a reaction mass is
prepared and sterilized through filtration.
|
Crystallizer designs come with the following advantages:
• Minimum space requirement
• Optimized Crystallizer design Range: Currently have executed projects with
2000 & 4000 liters capacity, however the volumes can be customized to user
requirements
• Floor-mounted structure . Mechanical/Magnetic - low shear Agitator (Top/Bottom
Mounted)
• Flexibility in configuration to meet the budgets
• Design conforms to ASME BPE and GAMP 5 guidelines
• Material of construction
• Parts in contact with the media: SS 316L
• Non-contact Parts: SS 304
• Internal surface Ra<=0.6, Electro-polished
• Double wet mechanical seal
• Designed for easy access during operation and routine maintenance
• PLC control system
• Configurable control loops
• Safety features to protect the batch in case of any component failure
•• The Crystallizer has provision for manual Sterilization-in-Place &
Cleaning-in-Place.
|
|
Batch
Bioinactivation System
• Flexible range
• Skid-mounted
• Agitator (Top/Bottom Mounted)
• Configuration to suit space restrictions
• Design conforms to ASME BPE and GAMP 5 guidelines
• Material of construction - SS 304
• Internal finish Ra<=0.8
• Designed for easy access during operation and routine maintenance
• PLC control system with SCADA
• Configurable control loops
• 100% redundancy for safety
Continuous Bioinactivation System
• Demonstrates up-to 62% Energy Efficiency as compared to a conventional model
• Completes a cycle in one-fourth the time as compared to a conventional model
Processes large volumes in a short span of time Is Customized to User
requirement
• Realizes a shorter payback period, achieved through Efficient Utility & Energy
Utilization Offers Reduced Space Utilization, Reduced man hours and increased
Productivity Adheres to ASME BPE and GAMP 5 design guidelines Offers
specifications as:
• Material of construction - SS 304
• Internal finish Ra <= 0.8
• PLC control system with SCADA
• Configurable control loops
• Is Designed for easy access during operation and routine maintenance
• Has 100% redundancy for safety .
|

Bioinactivation system caters to inactivation of biowaste generated at every
step of the biopharmaceutical production. BiOZEEN Bioinactivation system is
designed to inactivate these effluents using heat treatment mechanism. BiOZEEN
offers 2 types of bioinactivation products based on mode of operation namely:
•Batch Bioinactivation system •Continuous Bioinactivation system.
|

.All our modules are developed in association with the various industry experts
and are designed to bridge the gap between theoretical knowledge and its
practical industrial application.
Our programs include :
‧ Microbial Fermentation Technology
‧ Mammalian Cell Culture Technology
‧ Downstream Processing Technology
‧ Sterilization & Filtration Technology
‧ Bioprocess Engineering
‧ Facility and Utility Management ‧ Regulatory Affairs
‧ Process and Equipment Validation
‧ Operation, Design and Maintenance of Bioreactors, Fermentors and allied
Process Systems
‧ Technology Transfer and Process Development . |
Our
training programs cater to a wide range of participants from different walks
of the Biotechnology Sector namely:
• Training for Students
• Training for Industrial Personnel
BIOZEEN Training infrastructure -
• A provision to practice theoretical concepts in state-of-the-art Bio-Pilot
Laboratory occupying 8,000+sq ft. area over two floors with "what could go
wrong?" generators
• Upstream Processing - Fermentors and Bioreactors of 5L/ 40L/125L capacities to
facilitate growing of mammalian cell culture and microbial culture with
perfusion technology.
• Downstream Processing: Consisting of cell harvesting and cell disruption
systems, Chromatography, Tangential Flow Filtration systems - Micro, Ultra and
Diafiltration systems and integrity testing systems.
• Manufacturing Support: Buffer and media preparation systems, harvest system
and Sterilization systems - Autoclave and Dry Heat Sterilizer.
• Central clean Utilities Systems - WFI Generator, Purified water, CIP station,
Clean air-AHU systems and pure steam generators.
• The amenities supporting our training programs include cafeteria, library,
auditoriums to accommodate about 100 candidates.
|
|
Automation Technology enables monitoring, recording, analyzing and
controlling of entire plant operations (ranging from the equipment and Human
Machine Interface (HMI), MES levels, right up to interfacing with Enterprise
Resource Planning (ERP) systems) from a single work station to ensure ease in
batch control, assured batch reproducibility and audit compliance.
Our Automation Team at BiOZEEN ensures user friendly operations which are 21 CFR
Part 11 ready, in the highly challenging Pharmaceutical & Biopharmaceutical
production space.
Our capabilities include:
• Seamless automation & Integration of:
• Multiple unit operations: fermentation, Cleaning in Place, Sterilization in
Place, chromatography etc.
• Multiple processes: upstream, downstream and fill & finish
• 3rd party integration : seamless integration of equipment from multiple
vendors for multiple unit operations
• Retrofit
• Upgradation of systems .
|

Our capabilities include:
Seamless automation & Integration of:
• Multiple unit operations: fermentation, Cleaning in Place, Sterilization in
Place, chromatography etc.
• Multiple processes: upstream, downstream and fill & finish
• Seamless integration of equipment from multiple vendors for multiple unit
operations
• Retrofiting and Upgradation of systems.
|

With core capability in biologics/ biopharmaceutical processing, the Bioprocess
Team at BiOZEEN, delivers bioprocess solutions that ensure operational
efficiency, process integrity and optimized throughput – all in compliance with
the regulatory guidelines.
|
Bioprocess solutions or process design & engineering, with core capability
in biologics/biopharmaceutical processing, the Bioprocess Team at BIOZEEN,
delivers bioprocess solutions that ensure operational efficiency, process
integrity and optimized throughput - all in compliance with the regulatory
guidelines. Our competences which enable successful, repeatable and optimized
batch results include:
• Equipment Design with Piping and Instrumentation Diagrams, Pipe Sizing, Bill
of Material for Equipment and Piping, Selection of bought out components,
Generation of Valve Matrix and Sequential Flow Chartbr />
• Interconnection piping
• Process Scale Up Studies
& • Technology Transfer Analysis and Support
Our competences which enable successful, repeatable and optimized batch results
include: • Equipment Design with Piping and Instrumentation Diagrams, Pipe
Sizing, Bill of Material for Equipment and Piping, Generation of Valve Matrix
and Sequential Flow Chart • Interconnection piping • Vaccine and MABs Process
Support • Technology Transfer Support
|
|
The
filter validation services offered by BiOZEEN include:
• Filtration Train Optimization Study: to assess the series of filter devices
including pre-filtration and sterile filtration combination, with an objective
to ensure an optimized filtration train capable of providing maximum throughput
of the final sterile product without interference between batches.
• Compatibility Study:
plays a pivotal role in submissions pertaining to a new product market
authorization and in formulation experiments relevant to changes in product. It
is essential to thoroughly assess the chemical compatibility of the product
contact components with all equipment, filter devices and materials used in the
pharmaceutical manufacturing process, in order to avoid delays in the product
development process and deviations that could compromise the quality of the end
product. Filter integrity is evaluated post exposure to process fluid at process
conditions, as well as pre-determined worst case conditions that include;
simulated filter sterilization conditions (for devices), process fluid contact
time and process temperature - to determine that the pore size remains
unaffected. This is essential preliminary indication to reinforce the choice of
the membranes to be used in the filtration train.
• Product based Integrity Study:
Integrity of the filter is its Quality attribute. Sterilizing grade filters
require to be tested to assure its integrity pre- and post-filtration
process.Integrity values for the test filter are obtained by subjecting it to
real life situations - cleaning & sterilization-inplace, followed by integrity
testing under normal manufacturing conditions before establishing product wet
integrity values. BIOZEEN specialists also perform drug product based integrity
testing on filters to establish product integrity ratio values & product bubble
point ratio also.
• Bacterial Retention Study:
Validation of a sterilizing filtration process is critical since it is
impossible with currently available technology to measure the sterility of each
filled container; therefore, sterility assurance of the filtered product must be
achieved through validation of the filtration process, hence proving that
sterile filtrate is generated.As a part of this study, the filter is challenged
with Brevundimonas diminuta (ATCC 19146) under process conditions and
demonstrated by testing to produce a sterile filtrate. The size of B.diminuta is
critical for determining the retention characteristics of membrane filters. At
BIOZEEN the bacterial retention study is performed by trained microbiologists
who keep the size of the challenge organism to the minimum and as a
mono-dispersed suspension This creates the worst case challenge condition to
establish the retention capabilities of a SGF which can be correlated to the
product base integrity values.
•
Extractable & Leachable Study:
The primary function of filter devices is to remove unwanted contaminants from
pharmaceutical products. An ideal filtration device is non-interactive; it does
not adsorb any active ingredients from the drug, nor impart extractable into the
drug product. At BIOZEEN we to test the filtration device for its retention
characteristics, non-interactive ability and safety under minimum and maximum
operating conditions. Protein/Preservative Binding Study: Adsorption is a
mechanism, which causes the product to bind to the filter membrane; ideally, a
filter membrane should not adsorb formula components. Adsorptive filter
materials include, membrane, hardware & support materials. Adsorption tests are
performed at small scale & confirmed at large scale to ensure product
composition & concentration is not affected by such phenomenon taking into
consideration factors such as flow rate, product concentration, contact time,
preservative concentration, temperature & pH that can affect the level of
adsorption.
|

Method Development Study Microbiological Test Method Development is a time
consuming process. We at BIOZEEN are committed to deliver customized services,
ensuring compliance to regulatory needs.
Our team of experts would support you in understanding the Microbiology Method
Development necessities & Regulatory Requirement (USP/EP) associated with
testing of Finished Products, IPQC Samples & Raw materials. The services include
method development for performing
• Bio burden Testing & • Sterility Testing :
Sterile - filter validation services ‧ Filtration Train Optimization Studies
‧ Product Bubble Point Studies
‧ Filter Compatibility Studies
‧ Bacterial Retention Studies
‧ Filter Extractable studies
‧ Drug Product Filterability Studies
‧ Product Diffusion Studies Microbiology services
‧ Method Development for non sterile products
‧ Bioburden studies
‧ Sterility testing studies
‧ Bacterial Endotoxin studies
.
|
|